February 2025
FDA Approves Ketamine Nasal Spray for Treatment-Resistant Depression
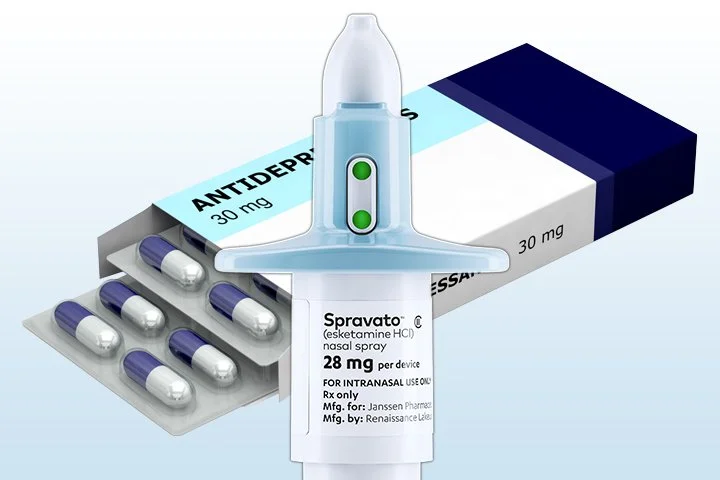
The FDA has approved Spravato (esketamine), a nasal spray, as a standalone treatment for treatment-resistant depression (TRD). Clinical trials have shown that esketamine, when used with an oral antidepressant, significantly reduces depressive symptoms within days—much faster than traditional antidepressants, which can take weeks or months to show results. This treatment is especially beneficial for those who haven’t responded to at least two previous antidepressants. While effective, esketamine must be closely monitored due to potential side effects, including dissociation and elevated blood pressure.
Dr. Neuman notes, "This is an exciting option for those who have not found success with other treatments. It's not for everyone, and like most therapies, the more you put into it, the more you get out. I'm hopeful insurance coverage will improve, as approval has been an issue, and many patients currently rely on compounding pharmacies for ketamine lozenges or nasal sprays."
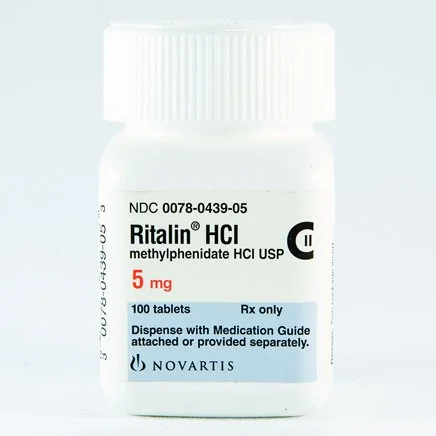
Methylphenidate (Ritalin) Safety During Pregnancy
A large new study involving 30,000 pregnancies found no significant increase in congenital anomalies or miscarriages in babies born to mothers treated with methylphenidate (Ritalin) or atomoxetine during pregnancy. These results were consistent with those of unexposed offspring or the general population.
Dr. Neuman notes, "While we generally aim to limit medication exposure during pregnancy, we also want to reduce stress and dysfunction for the mother and fetus. Deciding whether to continue or stop medication is a case-by-case discussion. This new study offers reassurance that either choice is likely safe."
GLP-1RA Reduces Risk of Cognitive Decline, Among Other Benefits
As you may have seen in past newsletters, Dr. Neuman is particularly interested in GLP-1 receptor agonists (GLP-1RAs) like semaglutide (WeGovy, Rybelsus, Ozempic) and tirzepatide (Mounjaro, Zepbound). A recent large meta-analysis compared GLP-1RA use to placebo and found these medications were linked to a reduced risk of cognitive decline, substance use, psychotic disorders, seizures, neurocognitive issues, and several other conditions. However, they also showed an increased risk of gastrointestinal disorders, hypotension, syncope, arthritis, kidney issues, and drug-induced pancreatitis. Overall, the benefits appear significant, though some risks remain.